Definitions Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol.
Overview of Physical Properties of Metals
Aug 10, 20221 molAl 26.98g Al and 26.98gAl 1mol Al (5.4.1) (5.4.1) 1 m o l A l 26.98 g A l a n d 26.98 g A l 1 m o l A l. The first conversion factor can be used to convert from mass to moles, and the second converts from moles to mass. Both can be used to solve problems that would be hard to do “by eye.”. Example 5.4.1 5.4. 1.

Source Image: zzfittings.com
Download Image
Molar Mass of an Element. According to the periodic table, the atomic mass of aluminum is 26.98 amu, copper is 63.55 amu, and carbon is 12.01 amu.Since 1 amu is only 1.674 × 10 −24 g, these masses would be way too small to measure on ordinary laboratory equipment. However, if we have 6.022 × 10 23 atoms, or 1 mole of each of these elements, we would have 26.98 g Al, 63.55 g Cu, and 12.01 g C.

Source Image: pubs.acs.org
Download Image
Chapter 3 Chemical Quantities (The Mole). The Mole 1 dozen = 1 gross = 1 ream = 1 mole = x mole Carbon = g Carbon 6 C ppt download Formula The molar mass of Al (Aluminium) is: 26.981 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Tool Overview: Molar Mass of Aluminium (Al) Solving for the atomic mass of Aluminium (Al) Need to know the atomic mass of a Aluminium molecule?

Source Image: youtube.com
Download Image
What Is The Molar Mass Of Aluminum
Formula The molar mass of Al (Aluminium) is: 26.981 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Tool Overview: Molar Mass of Aluminium (Al) Solving for the atomic mass of Aluminium (Al) Need to know the atomic mass of a Aluminium molecule? The periodic table shows that the atomic mass (rounded to two decimal points) of Al is 26.98, so 1 mol of Al atoms has a mass of 26.98 g. According to the periodic table, 1 mol of U has a mass of 238.03 g, so the mass of 2 mol is twice that, or 476.06 g. The mole concept can be extended to masses of formula units and molecules as well.
What is the molar mass of aluminum? – YouTube
The molar mass of aluminum is 26.982 g/mol. The molar mass of an element is the mass in grams of 1 mole of the element. It is determined by taking the atomic weight of the element on the periodic table, and writing it as g/mol. Aluminum Sulfate | Formula, Molar Mass & Properties – Video & Lesson Transcript | Study.com

Source Image: study.com
Download Image
SOLVED: please show the calculation of the molar mass of alum, report the unrounded value. The molar mass of aluminum is 26.982 g/mol. The molar mass of an element is the mass in grams of 1 mole of the element. It is determined by taking the atomic weight of the element on the periodic table, and writing it as g/mol.

Source Image: numerade.com
Download Image
Overview of Physical Properties of Metals Definitions Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol.

Source Image: blog.eaglegroupmanufacturers.com
Download Image
Chapter 3 Chemical Quantities (The Mole). The Mole 1 dozen = 1 gross = 1 ream = 1 mole = x mole Carbon = g Carbon 6 C ppt download Molar Mass of an Element. According to the periodic table, the atomic mass of aluminum is 26.98 amu, copper is 63.55 amu, and carbon is 12.01 amu.Since 1 amu is only 1.674 × 10 −24 g, these masses would be way too small to measure on ordinary laboratory equipment. However, if we have 6.022 × 10 23 atoms, or 1 mole of each of these elements, we would have 26.98 g Al, 63.55 g Cu, and 12.01 g C.

Source Image: slideplayer.com
Download Image
Molar Mass of Aluminum & Properties of Substance Element Aluminium (Al), Group 13, Atomic Number 13, p-block, Mass 26.982. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content . Periodic Table. Home; … Relative atomic mass The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and

Source Image: studybay.com
Download Image
Aluminium Oxide – Definition, Chemical and Physical Properties Formula The molar mass of Al (Aluminium) is: 26.981 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Tool Overview: Molar Mass of Aluminium (Al) Solving for the atomic mass of Aluminium (Al) Need to know the atomic mass of a Aluminium molecule?
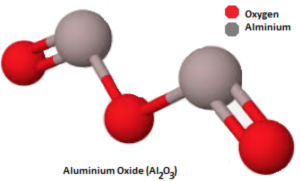
Source Image: infinitylearn.com
Download Image
Percentage Composition – ppt download The periodic table shows that the atomic mass (rounded to two decimal points) of Al is 26.98, so 1 mol of Al atoms has a mass of 26.98 g. According to the periodic table, 1 mol of U has a mass of 238.03 g, so the mass of 2 mol is twice that, or 476.06 g. The mole concept can be extended to masses of formula units and molecules as well.

Source Image: slideplayer.com
Download Image
SOLVED: please show the calculation of the molar mass of alum, report the unrounded value.
Percentage Composition – ppt download Aug 10, 20221 molAl 26.98g Al and 26.98gAl 1mol Al (5.4.1) (5.4.1) 1 m o l A l 26.98 g A l a n d 26.98 g A l 1 m o l A l. The first conversion factor can be used to convert from mass to moles, and the second converts from moles to mass. Both can be used to solve problems that would be hard to do “by eye.”. Example 5.4.1 5.4. 1.
Chapter 3 Chemical Quantities (The Mole). The Mole 1 dozen = 1 gross = 1 ream = 1 mole = x mole Carbon = g Carbon 6 C ppt download Aluminium Oxide – Definition, Chemical and Physical Properties Element Aluminium (Al), Group 13, Atomic Number 13, p-block, Mass 26.982. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content . Periodic Table. Home; … Relative atomic mass The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and