This video is about figuring out how to determine the hybridization of each element in its structure. Orbital hybridization is the concept of mixing atomic o
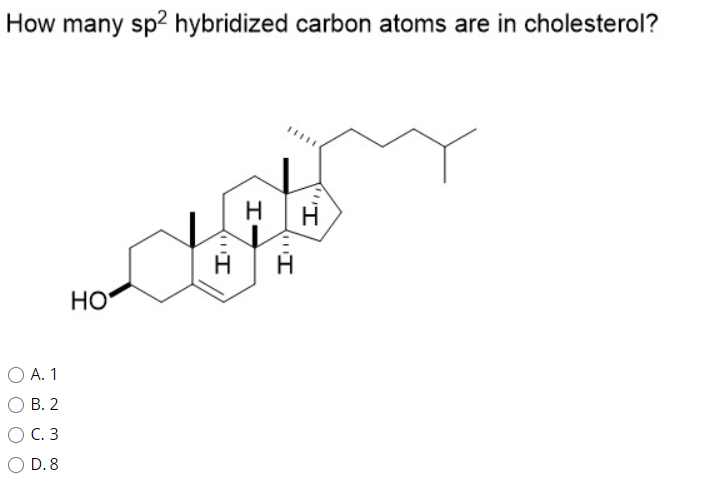
SOLVED: 7) How many sp2 hybridized atoms are in the following compound? 1 2 3
The three hybridized orbitals explain the three sigma bonds that each carbon forms. sp2 hybridization in etheneIn sp^2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of three sp^2 orbitals with one p-orbital remaining. The two carbon atoms form a sigma bond in the molecule by overlapping
Source Image: chegg.com
Download Image
Methyl amine. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s orbitals from hydrogens to form the two N-H sigma bonds. One of the sp 3 hybridized orbitals overlap with an sp 3 hybridized orbital from carbon to form the C-N sigma bond. The lone pair electrons on the nitrogen are contained in the last sp 3
Source Image: chegg.com
Download Image
My buddy said this is anti-aromatic I said it was non aromatic because sp2 hybridized atoms are required. Wouldn’t Fluorine be sp3? : r/chemhelp SUNY Potsdam Book: Organic Chemistry I (Walker) 2: Bonding and Molecular Structure 2.2: Hybrid orbitals Expand/collapse global location 2.2: Hybrid orbitals Page ID Table of contents Skills to Develop sp3 Hybrid orbitals and tetrahedral bonding

Source Image: toppr.com
Download Image
How Many Sp2 Hybridized Atoms Are In The Following Compound
SUNY Potsdam Book: Organic Chemistry I (Walker) 2: Bonding and Molecular Structure 2.2: Hybrid orbitals Expand/collapse global location 2.2: Hybrid orbitals Page ID Table of contents Skills to Develop sp3 Hybrid orbitals and tetrahedral bonding ALKENES AND sp 2 HYBRIDIZATION OF CARBON. We will now reproduce the sp 3 hybridization process for carbon, but instead of taking one s and three p orbitals to make four equivalent sp 3 orbitals, this time we’ll take only one s and two p orbitals to make three equivalent sp 2 orbitals, leaving one p orbital untouched. The process is shown below. As shown, the three resulting sp 2 orbitals are
How many sp2 and sp-hybridised carbon atoms are present respectively in the following compound?
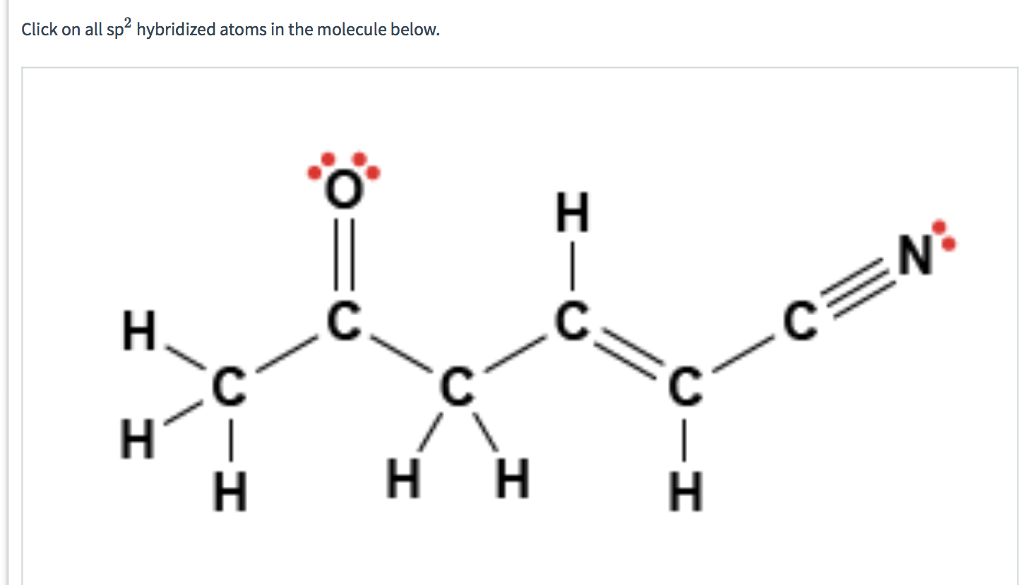
1. Some Simple Worked Examples Of The Hybridization Shortcut sp3 hybridization: sum of attached atoms + lone pairs = 4 sp2 hybridization: sum of attached atoms + lone pairs = 3 sp hybridization: sum of attached atoms + lone pairs = 2 Solved 2 How many sp2 hybridized atoms are in the following | Chegg.com
Source Image: chegg.com
Download Image
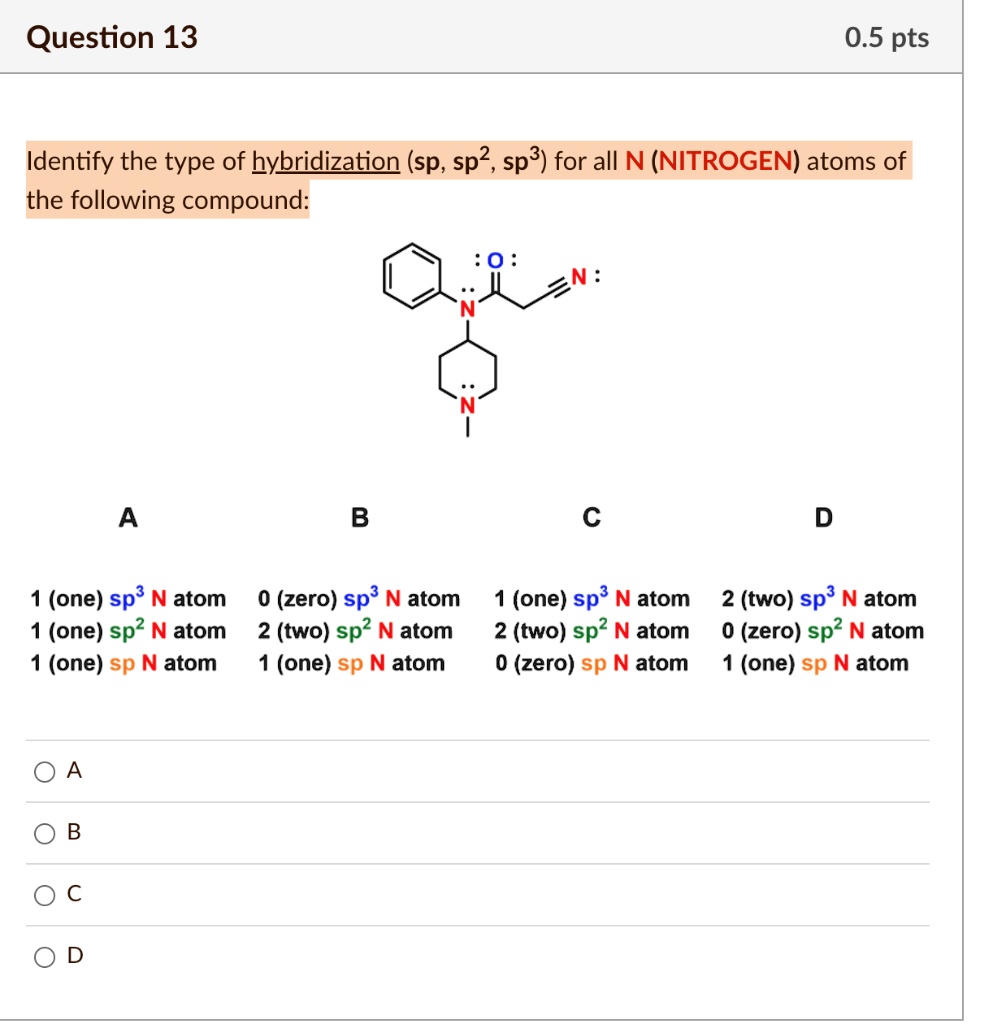
SOLVED: Question 13 0.5 pts Identify the type of hybridization (sp, sp2, sp3) for alI N (NITROGEN) atoms of the following compound: 0 A B c D 1 (one) sp3 N atom 1. Some Simple Worked Examples Of The Hybridization Shortcut sp3 hybridization: sum of attached atoms + lone pairs = 4 sp2 hybridization: sum of attached atoms + lone pairs = 3 sp hybridization: sum of attached atoms + lone pairs = 2
Source Image: numerade.com
Download Image
SOLVED: 7) How many sp2 hybridized atoms are in the following compound? 1 2 3 This video is about figuring out how to determine the hybridization of each element in its structure. Orbital hybridization is the concept of mixing atomic o

Source Image: numerade.com
Download Image
My buddy said this is anti-aromatic I said it was non aromatic because sp2 hybridized atoms are required. Wouldn’t Fluorine be sp3? : r/chemhelp Methyl amine. The nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s orbitals from hydrogens to form the two N-H sigma bonds. One of the sp 3 hybridized orbitals overlap with an sp 3 hybridized orbital from carbon to form the C-N sigma bond. The lone pair electrons on the nitrogen are contained in the last sp 3

Source Image: reddit.com
Download Image
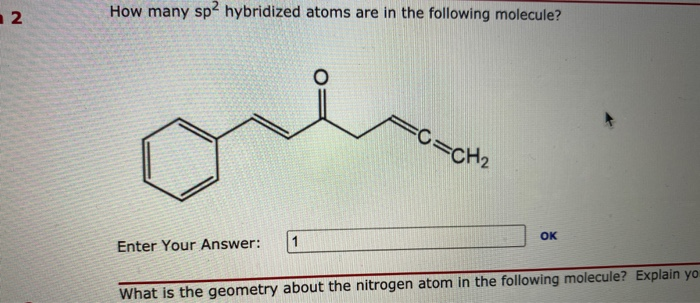
Solved How many sp2 hybridized carbons are present in | Chegg.com The four sp3 –hybridized orbitals arrange in a tetrahedral geometry and make bonds by overlapping with the s orbitals of four hydrogens: This explains the symmetrical geometry of methane (CH 4) where all the bonds have the same length and bond angle.
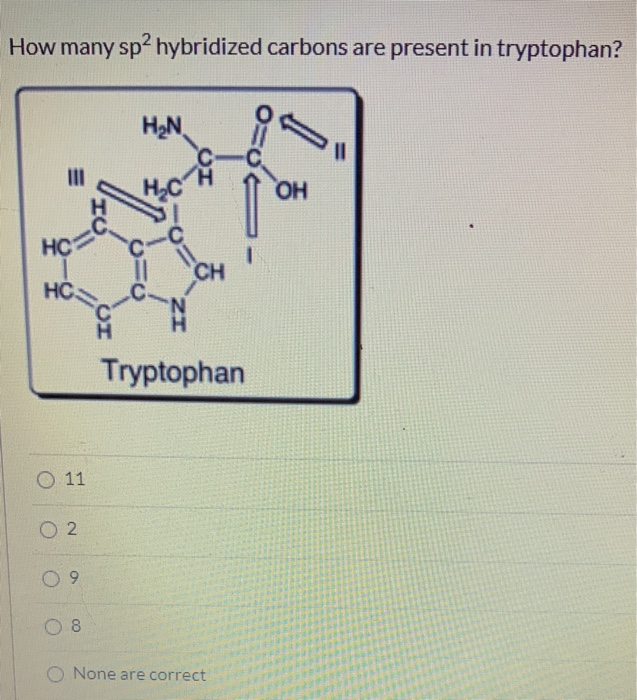
Source Image: chegg.com
Download Image
How does sp2 hybridized carbon differ from sp3? | Socratic SUNY Potsdam Book: Organic Chemistry I (Walker) 2: Bonding and Molecular Structure 2.2: Hybrid orbitals Expand/collapse global location 2.2: Hybrid orbitals Page ID Table of contents Skills to Develop sp3 Hybrid orbitals and tetrahedral bonding
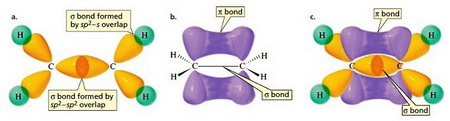
Source Image: socratic.org
Download Image
How many sp^2 hybridised carbon atoms and pi bonds respectively are pr ALKENES AND sp 2 HYBRIDIZATION OF CARBON. We will now reproduce the sp 3 hybridization process for carbon, but instead of taking one s and three p orbitals to make four equivalent sp 3 orbitals, this time we’ll take only one s and two p orbitals to make three equivalent sp 2 orbitals, leaving one p orbital untouched. The process is shown below. As shown, the three resulting sp 2 orbitals are
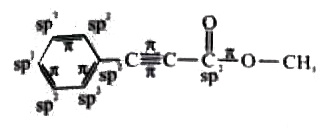
Source Image: doubtnut.com
Download Image
SOLVED: Question 13 0.5 pts Identify the type of hybridization (sp, sp2, sp3) for alI N (NITROGEN) atoms of the following compound: 0 A B c D 1 (one) sp3 N atom
How many sp^2 hybridised carbon atoms and pi bonds respectively are pr The three hybridized orbitals explain the three sigma bonds that each carbon forms. sp2 hybridization in etheneIn sp^2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of three sp^2 orbitals with one p-orbital remaining. The two carbon atoms form a sigma bond in the molecule by overlapping
My buddy said this is anti-aromatic I said it was non aromatic because sp2 hybridized atoms are required. Wouldn’t Fluorine be sp3? : r/chemhelp How does sp2 hybridized carbon differ from sp3? | Socratic The four sp3 –hybridized orbitals arrange in a tetrahedral geometry and make bonds by overlapping with the s orbitals of four hydrogens: This explains the symmetrical geometry of methane (CH 4) where all the bonds have the same length and bond angle.