The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by `L` i
Ternary Phase Diagram of Water/Bio-Oil/Organic Solvent for Bio-Oil Fractionation | Energy & Fuels
Thus the boiling point of a solution is always greater than that of the pure solvent. We can see why this must be true by comparing the phase diagram for an aqueous solution with the phase diagram for pure water (Figure \(\PageIndex4\)). The vapor pressure of the solution is less than that of pure water at all temperatures.

Source Image: pubs.acs.org
Download Image
Draw a phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. … Draw a phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution.

Source Image: researchgate.net
Download Image
Measuring Freezing Point Decrease of Water Solutions As a Way to Determine the Molecular Mass of the Solute : 5 Steps (with Pictures) – Instructables VIDEO ANSWER: Alright, all right. You were asked to compare the phase diagram for a pure solvent versus a solution. The way face diagram is constructed is you’re looking at the effects of pressure and temperature on different states of matter. It was
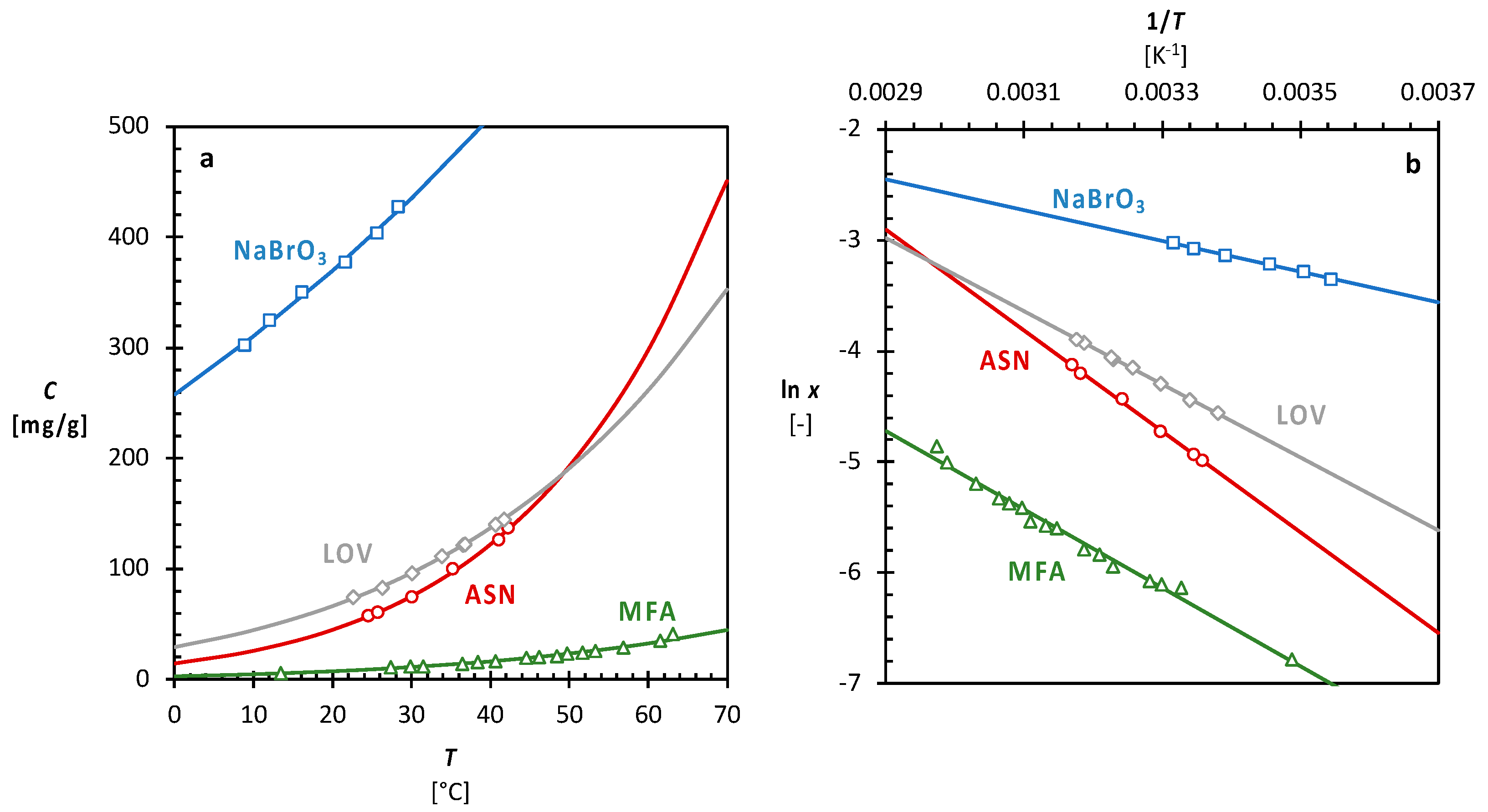
Source Image: mdpi.com
Download Image
Label The Phase Diagram Of Pure Solvent And A Solution.
VIDEO ANSWER: Alright, all right. You were asked to compare the phase diagram for a pure solvent versus a solution. The way face diagram is constructed is you’re looking at the effects of pressure and temperature on different states of matter. It was Effect of Solute on Phase Diagram of Water SOLID LIQUID GAS Pure solvent Freezing point of solution Freezing point of water Boiling point of water Boiling point of solution 1 atm Pressure ΔTf ΔTb ΔP Temperature. Title: phase_diagram_solvent_solution.ai Author: Joy Walker Created Date:
Crystals | Free Full-Text | Phase Diagram Determination and Process Development for Continuous Antisolvent Crystallizations
Highlights Learning Objectives By the end of this section, you will be able to: Explain the construction and use of a typical phase diagram Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties Describe the supercritical fluid phase of matter Does a Binary Phase Diagram Exist for a Solvent Containing a Single Solute Molecule? Case of a Neutral Solute Molecule in Ethanol | The Journal of Physical Chemistry C

Source Image: pubs.acs.org
Download Image
D4 Phase Diagrams for Pure Substances – YouTube Highlights Learning Objectives By the end of this section, you will be able to: Explain the construction and use of a typical phase diagram Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties Describe the supercritical fluid phase of matter
Source Image: m.youtube.com
Download Image
Ternary Phase Diagram of Water/Bio-Oil/Organic Solvent for Bio-Oil Fractionation | Energy & Fuels The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by `L` i

Source Image: pubs.acs.org
Download Image
Measuring Freezing Point Decrease of Water Solutions As a Way to Determine the Molecular Mass of the Solute : 5 Steps (with Pictures) – Instructables Draw a phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. … Draw a phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution.

Source Image: instructables.com
Download Image
Does a Binary Phase Diagram Exist for a Solvent Containing a Single Solute Molecule? Case of a Neutral Solute Molecule in Ethanol | The Journal of Physical Chemistry C A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we’ll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.

Source Image: pubs.acs.org
Download Image
SOLVED: Consider the data in the phase diagram for a pure solvent in comparison to a solution of that solvent Then, choose the answer below that is FALSE Pure solvent Solution atm VIDEO ANSWER: Alright, all right. You were asked to compare the phase diagram for a pure solvent versus a solution. The way face diagram is constructed is you’re looking at the effects of pressure and temperature on different states of matter. It was
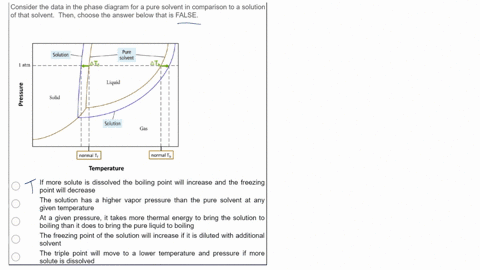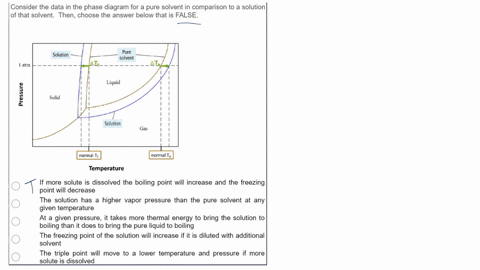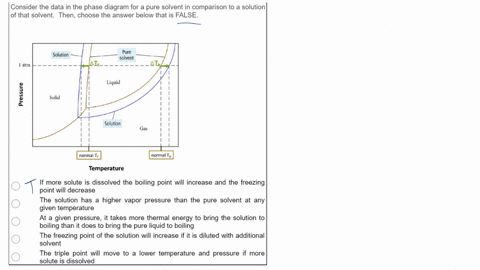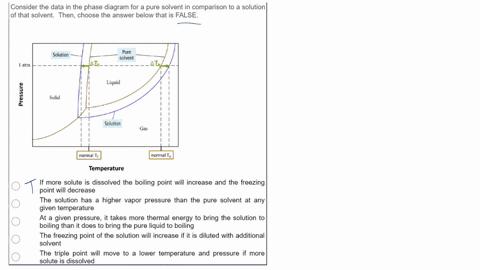
Source Image: numerade.com
Download Image
328 Water Phase Diagram Images, Stock Photos, 3D objects, & Vectors | Shutterstock Effect of Solute on Phase Diagram of Water SOLID LIQUID GAS Pure solvent Freezing point of solution Freezing point of water Boiling point of water Boiling point of solution 1 atm Pressure ΔTf ΔTb ΔP Temperature. Title: phase_diagram_solvent_solution.ai Author: Joy Walker Created Date:

Source Image: shutterstock.com
Download Image
D4 Phase Diagrams for Pure Substances – YouTube
328 Water Phase Diagram Images, Stock Photos, 3D objects, & Vectors | Shutterstock Thus the boiling point of a solution is always greater than that of the pure solvent. We can see why this must be true by comparing the phase diagram for an aqueous solution with the phase diagram for pure water (Figure \(\PageIndex4\)). The vapor pressure of the solution is less than that of pure water at all temperatures.
Measuring Freezing Point Decrease of Water Solutions As a Way to Determine the Molecular Mass of the Solute : 5 Steps (with Pictures) – Instructables SOLVED: Consider the data in the phase diagram for a pure solvent in comparison to a solution of that solvent Then, choose the answer below that is FALSE Pure solvent Solution atm A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we’ll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.